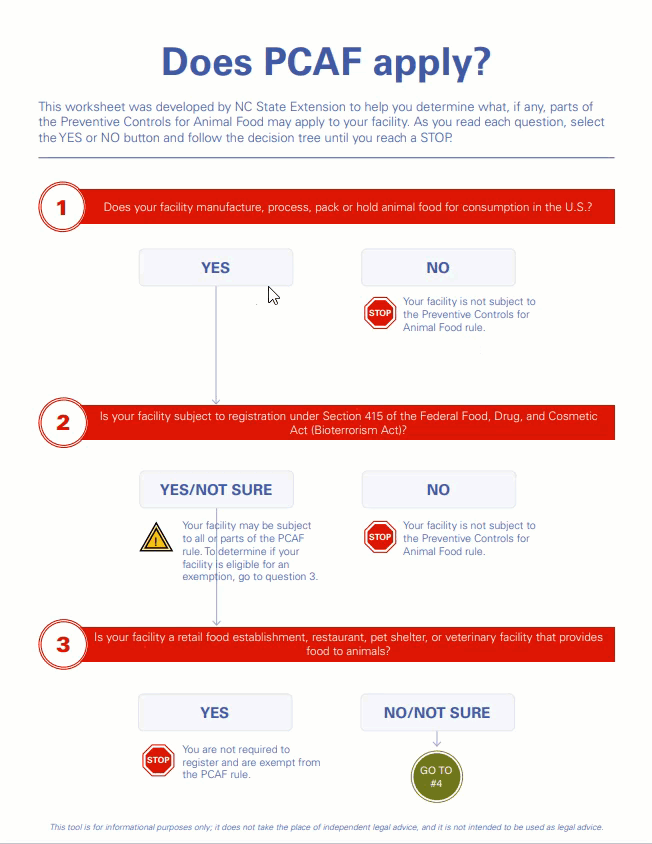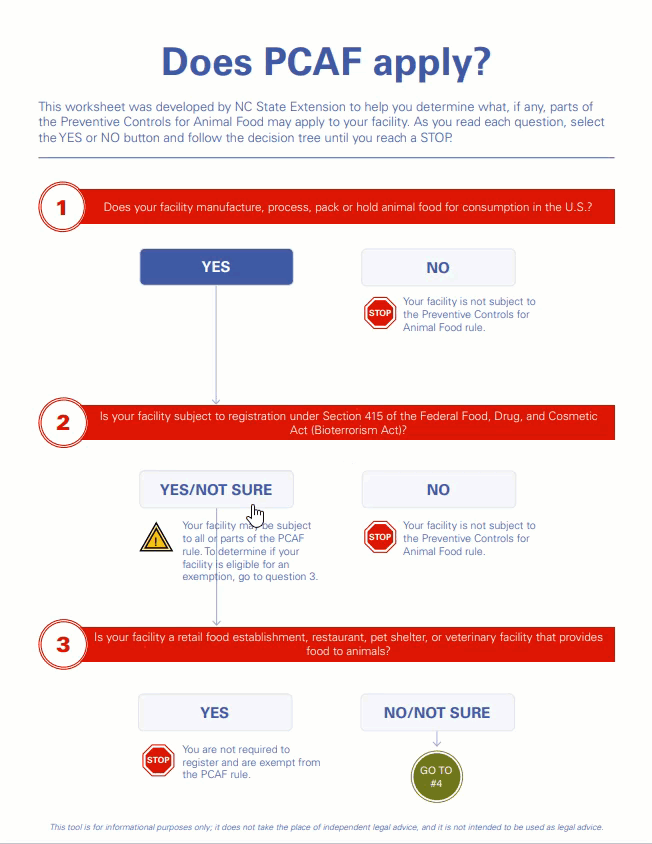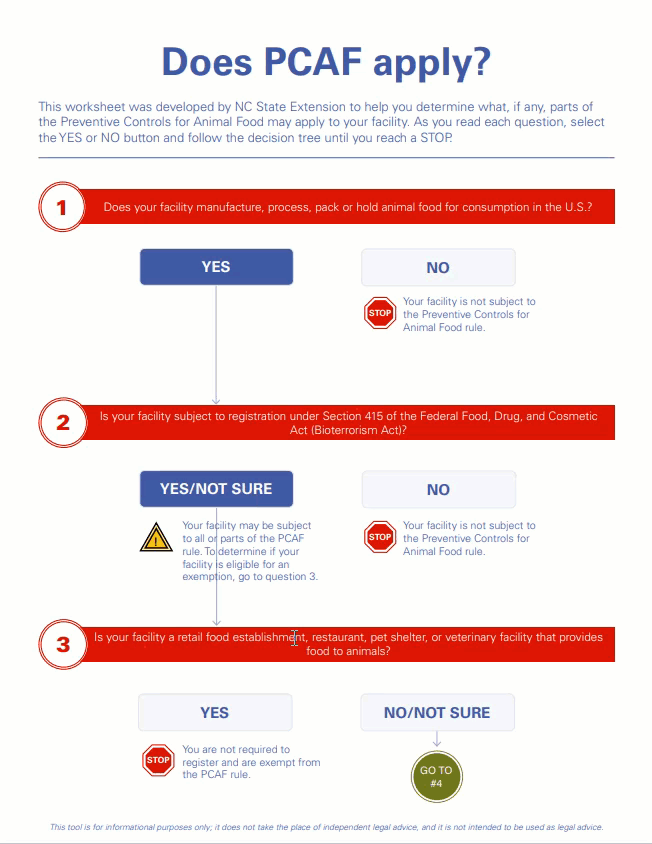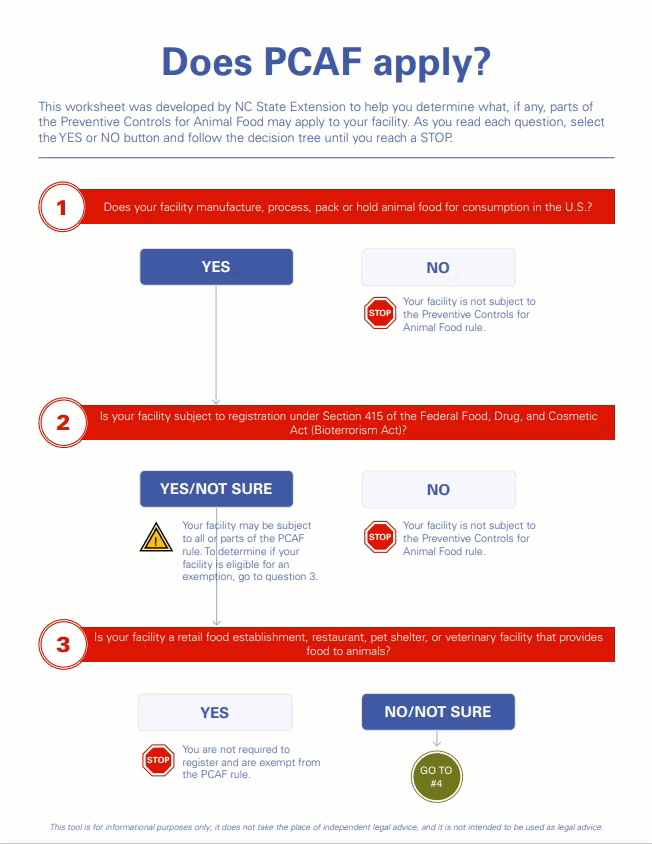
This certificate confirms that the above company is registered (as provided in registration details) with U.S Food and Drug Admi
A bill to amend the Federal Food, Drug, and Cosmetic Act with respect to the scope of new chemical exclusivity. (2021; 117th Congress S. 415) - GovTrack.us
The Safety and Regulation of Chickpeas, Lentils, and Field Peas in Farming and Post-Harvest Operations
Guidance for Industry: Registration of Food Facilities: What You Need to Know About the FDA Regulation; Small Entity Compliance

Guidance for Industry What You Need To Know About Registration of Food Facilities Small Entity Compliance Guide - PDF Free Download